

Validation Management Systems (VMS) Software
What is Validation Management Systems Software?
A Validation Management System is a digital software solution that performs data verification and data validation. This allows organizations to perform, maintain, and uphold unified processes for creating and executing all validation activities across multiple sites, platforms and countries inclusive of regulatory guidelines.
Common Features
- Forward and Reverse Validation Traceability
- Risk Management
- Compliance Management
- Automated Testing
- Document Management & Traceability
- Process Integration
- Audit, Reporting & Compliance
- Change Control
- Continuous Improvement
- Collaboration Tools
Our Methodology
Composite Score
Vendors are ranked by the composite score, which is comprised of feature, capability, relationship, and overall satisfaction scores while factoring in the volume and recency of reviews.
CX Score
The CX score is comprised of the emotional footprint metrics, balanced by metrics related to the overall value of the software, while factoring in the volume and recency of reviews.
Data Quadrant
SoftwareReviews collects user insights that help organizations more effectively choose software that meets their needs, measure business value, and improve selection.
Rankings, results, and positioning on SoftwareReviews reports are based entirely on end-user feedback solicited from a proprietary online survey engine.
Leaders
Products that resonate strongest in the market, balancing features with a great user experience.
Product Innovators
Products that emphasize product features, gaining strong recommendations from their customers.
Service Stars
Products that emphasize a good user experience and build strong relationships with customers.
Challengers
Products that are strong performers in some areas and trail in others. Often up-and-coming vendors.

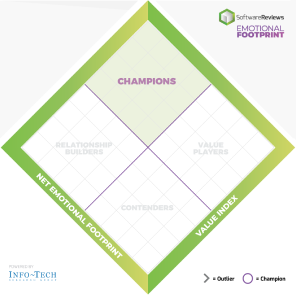
Emotional Footprint
SoftwareReviews collects user insights that help organizations more effectively choose software that meets their needs, measure business value, and improve selection.
Rankings, results, and positioning on SoftwareReviews reports are based entirely on end-user feedback solicited by a proprietary online survey engine.
Champions
Products that resonate highly with users at an emotional level. Users have specific emotional views towards these products like love, trust, and effectiveness.
Relationship Builders
Products that are bulletproof and focus on fulfilling core needs with steady support, not the latest feature.
Value Players
Products that succeed on cost and service effectiveness with users.
Contenders
Products seen as good in some areas and trailing in others. Users look to these for innovation at the edge but aren't fully committed.
Write a Review to receive up to a $10 Gift Card*
*After you complete our short 5-6 minute survey, we will happily provide you with your choice of reward up to $10 based on available options for your region.
Write a ReviewGet Instant Access<br>to this Report
Get Instant Access
to this Report
Unlock your first report with just a business email. Register to access our entire library.
© 2025 SoftwareReviews.com. All rights reserved.
